bonding quizlet|what is channel bonding quizlet : iloilo Q-Chat. Study with Quizlet and memorize flashcards containing terms like Octet . Resultado da HOT NEW. 10 18,4K. Vitoria Beatriz - Rabuda Vitória Ganhou Duas Rolas de Presente Natalino Ñaumñaum. 23 16,9K. T. Safada Vitória Beatriz .
0 · what is channel bonding quizlet
1 · polar covalent bond quiz
2 · metallic bonding quizlet
3 · introduction to chemical bonding quizlet
4 · human bonding quizlet
5 · grounding and bonding quizlet
6 · covalent bonding quizlet
7 · covalent and ionic bonding quizlet
Resultado da Build for Top, Platinum+. Patch 14.4. The Gnar build for Top is Sterak's Gage, Black Cleaver, and Trinity Force. This LoL Gnar guide for Top at .
bonding quizlet*******First, take the absolute value of the electronegativity difference between the two atoms in the bond. If electronegativity difference is >2.0, bond is ionic. If electronegativity difference is 0.5 - 2.0, bond is polar covalent. If electronegativity difference is <0.5, bond is non .Molecules. A double covalent bond is the sharing of a total of how many .bonding quizlet what is channel bonding quizletStudy with Quizlet and memorize flashcards containing terms like Define: Chemical .what is channel bonding quizletQ-Chat. Study with Quizlet and memorize flashcards containing terms like Octet .
18 terms. Christian_Cal. Preview. vocab. 28 terms. jess666667. Preview. Study with .Molecules. A double covalent bond is the sharing of a total of how many electrons? 4. The lines on the drawing of caffeine indicate: Covalent bonds. Study with Quizlet and .Study with Quizlet and memorize flashcards containing terms like Define: Chemical Bond, Define: Ionic Bond, Define: Chemical Formula and more.
Q-Chat. Study with Quizlet and memorize flashcards containing terms like Octet Rule, Lewis Dot structure, Ionic Bond and more.18 terms. Christian_Cal. Preview. vocab. 28 terms. jess666667. Preview. Study with Quizlet and memorize flashcards containing terms like When an atom bonds to another atom, it .Quiz 1. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit .What is this type of bond called. Double covalent. Triple Covalent. Polar covalent. Quadruple covalent. 62. Multiple Choice. Edit. 30 seconds. 1 pt. Atoms gain, lose or .A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic .
Example 9.2.1 9.2. 1: Sodium Chloride. For example, in the reaction of Na (sodium) and Cl (chlorine), each Cl atom takes one electron from a Na atom. Therefore .
Bonding. Ionic Bonding. Click the card to flip 👆. A bond between metals and non-metals. The metal will donate electrons to the non-metal forming ions. Ions are attracted towards one another via electrostatic forces. Click the card to flip 👆. 1 / 36.Terms in this set (5) Hydrogen Bond Definition. attraction between a highly electronegative atom in one polar bond and a slightly positive hydrogen atom in another polar bond. Qualities that water has due to the hydrogen bonds. high heat capacity. high heat of evaporation. high cohesion surface tension. excellent solven for other polar molecules.
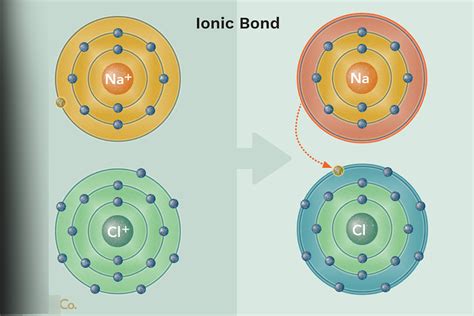
Study with Quizlet and memorize flashcards containing terms like A solid compound in a sealed container was kept at a very low temperature in a freezer. When placed at room temperature, the substance quickly turned into a liquid. This compound is most likely which of the following?, Which type of bond will form between two chlorine .
Ionic Bond. Chemical Formula. Diatomic Molecule. Lewis Structure. Molecule. Cation. Anion. metallic bond. Study with Quizlet and memorize flashcards containing terms like Valence Electrons, Covalent Bond, Ionic Bond and more.
Water is able to form hydrogen bonds because the oxygen has lone pairs on it - these can form a hydrogen bond with the H on another water molecule - as it is electron deficient so by bonding it will have a full outer shell. Water and ammonia hydrogen bonding. Hydrogen bonding between a molecule of water and a molecule of ammonia - the lone .

1. add number of valence e- from central atom and number of e- contributed by bonding. 2. add 1 if negative ion. subtract 1 if positive ion. 3. divide by 2. 4. identify which bonding. Enthalpy. The change of heat energy measured under a constant pressure. Heating a substance. More heat, particles move more, expands.A bond between non-metals where they share a pair of electrons. Where one atom donates a lone pair of electrons to an atom that is electron deficient. Also known as dative covalent bonding. Layers of positive metal ions in a sea of delocalised electrons. Allowing the metal to be conducting at a solid state.
Study with Quizlet and memorize flashcards containing terms like Which is the best metal to use in an alloy to increase its electrical conductivity? A. Al B. Cu C. Fe D. Sb, Which element is likely to be the best conductor of electricity? A. copper B. lithium C. iodine D. silicon, Which is most likely the result of millions of metal atoms crowding together so .
Study with Quizlet and memorize flashcards containing terms like Metallic bonds are responsible for many properties of metals, such as conductivity. Why is this possible?, Between which types of elements do ionic bonds occur, and how do electrons act within the bond?, Which kind of bond would occur between sodium (Na, Group IA) and chlorine .A covalent bond in which electrons are shared unequally. Metallic bond. An attraction between a positive metal ion and the electrons surrounding it. Alloy. A mixture of two or more elements, at least one of which is a metal. All about atoms and how they bond. Learn with flashcards, games, and more — for free.2. Draw O first, both with 2e- from Sulfur. 3. Sulfur will only need 2e so draw it from one O as only in pairs. - bond by which a lone pair of electrons shares its pair with an atom that is electron deficient. - bond between metals. - Consists of positive ions dispersed in a sea of delocalised electrons.bonding quizleta covalent bond produced by sharing one pair of electrons between two atoms. Structural formula. a formula that indicates the exact, number, and types of atoms present in a molecule and how they are bonded together. Triple bond. a covalent bond produced by sharing 3 pairs of electrons between atoms. Unshared pair.
O código do saque de aniversário já significa que o depósito do FGTS foi feito e estará disponível no dia seguinte a data informada no extrato. A solicitação de transferência da . Ver mais
bonding quizlet|what is channel bonding quizlet